Ophthalmic Drugs Market Expected To Grow US$ 85.6 Billion By 2033, Driven by 8.3% CAGR
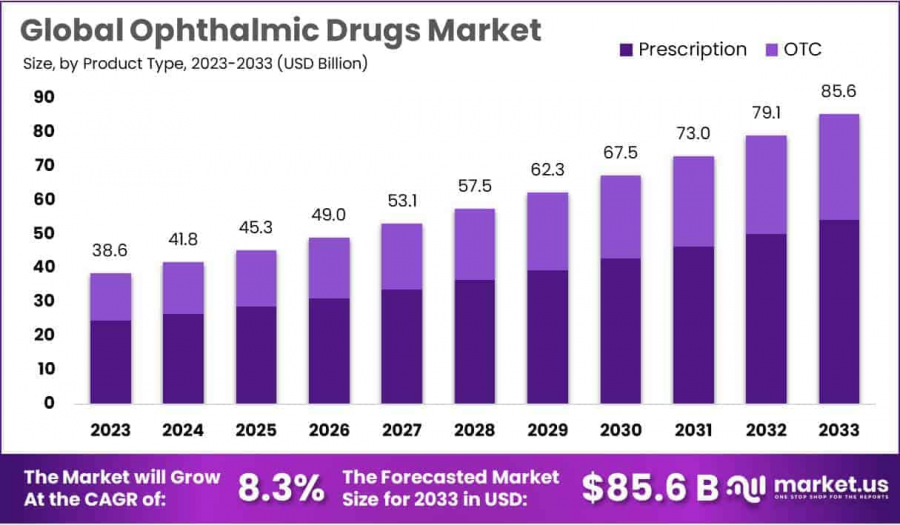
Global Ophthalmic Drugs Market size is expected to be worth around USD 85.6 Billion by 2033, from USD 38.6 Billion in 2023, growing at a CAGR of 8.3%
NEW YORK CITY, NY, UNITED STATES, January 23, 2025 /EINPresswire.com/ -- Report Overview
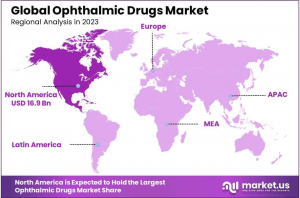
Global Ophthalmic Drugs Market size is expected to be worth around USD 85.6 Billion by 2033, from USD 38.6 Billion in 2023, growing at a CAGR of 8.3% during the forecast period from 2024 to 2033. With a market share over 44%, North America held a strong lead in 2023, reaching US$ 16.9 Billion in revenue.
The global ophthalmic drugs market is experiencing significant growth, driven by the increasing prevalence of eye disorders such as glaucoma, macular degeneration, and diabetic retinopathy. Advances in pharmaceutical formulations and drug delivery technologies have further propelled market expansion, offering innovative solutions for effective eye care.
Key therapeutic categories in the market include anti-glaucoma drugs, anti-inflammatory drugs, anti-infective drugs, and dry eye medications. With the growing aging population and rising incidences of chronic conditions affecting vision, the demand for effective ophthalmic treatments continues to rise.
The development of novel drug delivery methods, such as sustained-release implants and topical eye drops with enhanced bioavailability, is transforming patient care by improving treatment adherence and outcomes. Additionally, the integration of advanced diagnostic technologies facilitates early detection, which significantly boosts treatment success rates.
North America dominates the ophthalmic drugs market due to high healthcare spending, advanced healthcare infrastructure, and widespread adoption of innovative treatments. However, Asia-Pacific is emerging as a promising region, supported by increasing healthcare access and rising awareness about eye health.As the market evolves, investments in research and development remain a focal point for pharmaceutical companies, aiming to address unmet clinical needs and expand their product portfolios in ophthalmology.
Unlock Competitive Advantages With Our PDF Sample Report https://market.us/report/ophthalmic-drugs-market/request-sample/
Key Takeaways
• Market Projection: The ophthalmic drugs market is projected to reach USD 85.6 billion by 2033, growing at a robust CAGR of 8.3% from 2024 to 2033.
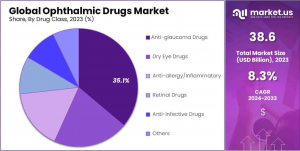
• Product Type Dominance: Prescription drugs accounted for 63.5% of the market share in 2023, fueled by the growing prevalence of AMD and diabetic retinopathy.
• Drug Class Dominance: Anti-glaucoma drugs held a 31.9% market share in 2023, driven by the increasing global incidence of glaucoma.
• Dosage Form Dominance: Eye drops led the market with a 41.8% share in 2023, supported by their OTC availability, ease of use, and frequent new product launches.
• Route of Administration Dominance: Topical administration dominated the market with 57% in 2023, preferred for its ease of self-administration, non-invasiveness, and patient adherence.
• Distribution Channel Dominance: Hospital pharmacies captured a 60.4% market share in 2023 due to the high prevalence of chronic eye diseases, while online pharmacies showed the fastest growth.
• Regional Analysis: North America led the market with a 44% share in 2023, attributed to the rising prevalence of eye disorders, advancements in technology, and a growing elderly population.
How Artificial Intelligence (AI) is Changing the Ophthalmic Drugs Market
Artificial Intelligence (AI) is revolutionizing the ophthalmic drugs market by enhancing diagnostic capabilities and expediting drug discovery processes. AI algorithms, particularly deep learning models, can analyze complex ocular images to detect diseases such as diabetic retinopathy and age-related macular degeneration with high accuracy. This facilitates early intervention and personalized treatment plans, improving patient outcomes.
Moreover, AI accelerates the drug development pipeline by predicting potential drug candidates and assessing their efficacy and safety profiles efficiently. The integration of AI into ophthalmology not only streamlines clinical workflows but also reduces costs associated with traditional drug development methodologies.
Market Segments:
Product Type
• Prescription Drugs
• OTC Drugs
Drug Class
• Anti-glaucoma Drugs
• Dry Eye Drugs
• Anti-allergy/Inflammatory
• Retinal Drugs
• Anti-infective Drugs
• Other Drug classes
Dosage Form
• Gels
• Eye Solutions & Suspensions
• Capsules and Tablets
• Eye Drops
• Ointments
Route of Administration
• Topical
• Local Ocular
• Systematic
Distribution Channel
• Hospital Pharmacies
• Online Pharmacies
• Other Distribution Channels
Distribution Channel
Hospital Pharmacies
Online Pharmacies
Other Distribution Channels
Buy This Premium Research Report@ https://market.us/purchase-report/?report_id=102423
Ophthalmic Drugs Market Analysis
• Driver: The increasing prevalence of ocular diseases globally serves as a primary driver for the ophthalmic drugs market. Conditions such as glaucoma, cataracts, and macular degeneration are becoming more common, particularly among the aging population. This rise in eye-related health issues necessitates the development and availability of effective ophthalmic medications to manage and treat these conditions, thereby propelling market growth.
• Trend: A notable trend in the ophthalmic drugs market is the shift towards preservative-free formulations. Preservatives in eye drops can cause adverse reactions in some patients, leading to discomfort and non-compliance. The development of preservative-free ophthalmic solutions addresses these concerns, offering safer alternatives and enhancing patient adherence to treatment regimens. ([U.S. Food and Drug
• Restraint: Stringent regulatory requirements pose a significant restraint in the ophthalmic drugs market. The U.S. Food and Drug Administration (FDA) mandates rigorous quality control measures for ophthalmic products, including sterility and stability standards. Compliance with these regulations can be challenging and time-consuming for manufacturers, potentially delaying product launches and increasing development costs.
• Opportunity: The integration of advanced drug delivery systems presents a substantial opportunity in the ophthalmic drugs market. Innovations such as nanotechnology-based delivery methods can improve the bioavailability and therapeutic efficacy of ophthalmic drugs. These advancements enable targeted and sustained drug release, minimizing side effects and enhancing patient outcomes.
Regional Analysis of the Ophthalmic Drugs Market
North America
In 2023, North America maintained a leading position in the ophthalmic drugs market, accounting for over 44% of the global share, with a market value of approximately USD 16.9 billion. This dominance is attributed to several factors:
- Prevalence of Eye Diseases: The region experiences a high incidence of ocular conditions, including age-related macular degeneration (AMD), diabetic retinopathy, and glaucoma. For instance, an estimated 20 million Americans are affected by AMD, with about 1.5 million facing vision-threatening forms of the disease.
- Technological Advancements: Continuous improvements in ophthalmic technology, such as the development of advanced imaging techniques and innovative drug delivery systems, have enhanced diagnostic and therapeutic capabilities. The National Eye Institute (NEI) has been instrumental in funding research that paves the way for new treatments, particularly for conditions like dry AMD.
- Aging Population: The increasing number of elderly individuals contributes to a higher demand for ophthalmic care, as aging is a significant risk factor for many eye diseases. The National Institutes of Health (NIH) highlights that the prevalence of eye diseases is expected to rise with the aging U.S. population.
- Regulatory Support: The U.S. Food and Drug Administration (FDA) actively facilitates the approval and monitoring of ophthalmic drugs and devices, ensuring timely access to safe and effective treatments. In 2023, the FDA approved 55 novel drugs, reflecting a commitment to advancing therapeutic options.
Asia-Pacific
The Asia-Pacific region is projected to experience significant growth in the ophthalmic drugs market during the forecast period. Key factors driving this expansion include:
- Rising Incidence of Ocular Diseases: An increase in conditions such as cataracts, glaucoma, and diabetic retinopathy is observed, partly due to lifestyle changes and an aging demographic. The World Health Organization (WHO) emphasizes the need for integrated people-centered eye care to address these challenges.
- Enhanced Awareness and Access: Growing awareness of eye health and improved access to healthcare services have led to higher diagnosis and treatment rates. The WHO's action plan for the South-East Asia region aims for a 40-percentage point increase in effective coverage of refractive error and a 30-percentage point increase in effective coverage of cataract surgery by 2030.
- Government Initiatives: Efforts to eliminate diseases like trachoma by 2025 and to ensure that at least 80% of people with diabetes are regularly screened for retinopathy demonstrate a strong commitment to eye health.
- Local Industry Participation: Regional companies are engaging in strategic initiatives to develop and market novel treatment options, fostering market growth. Collaborations and investments in research and development are contributing to the availability of advanced ophthalmic therapies.
Key Market Players:
• Pfizer Inc.
• Hoffmann-La Roche Ltd
• Bayer AG
• Bausch Health Companies Inc.
• Merck & Co., Inc.
• Novartis AG
• Santen Pharmaceutical Co. Ltd.
• Sun Pharmaceutical Industries Ltd.
• Regeneron Pharmaceuticals Inc.
• Coherus Biosciences, Inc.
• Alcon
• Other Key Players
Recent Developments in Ophthalmic Drugs
- In 2023, the FDA approved Byooviz (ranibizumab-nuna), the first biosimilar to Lucentis, for treating several eye conditions, including neovascular (wet) age-related macular degeneration.
- The FDA released updated guidance on quality considerations for topical ophthalmic drug products, emphasizing the importance of in vitro drug release testing and appropriate design of container closure systems to ensure product safety and efficacy.
Emerging Trends in Ophthalmic Drugs
- The adoption of in vitro drug release/dissolution testing as a quality control strategy is gaining traction. This approach allows for a more efficient assessment of drug release characteristics, contributing to the development of safer and more effective ophthalmic therapies.
- There is a growing focus on the design, delivery, and dispensing features of container closure systems (CCSs) for ophthalmic products. Properly designed CCSs are crucial to prevent contamination and ensure the stability and sterility of ophthalmic drugs throughout their shelf life.
Use Cases of Ophthalmic Drugs
- Antimicrobial eye drops are prescribed to treat bacterial infections such as conjunctivitis, effectively reducing infection rates. These medications help in alleviating symptoms and preventing the spread of infection.
- Anti-inflammatory ophthalmic solutions are used post-surgery to alleviate inflammation, enhancing recovery times. By reducing inflammation, these drugs aid in faster healing and improve patient comfort during the postoperative period.
- Medications like cyclosporine ophthalmic emulsion have been approved to increase tear production in patients with dry eye disease, addressing a condition that affects millions globally. This treatment improves lubrication of the eyes, reducing discomfort and the risk of further complications.
Get More Related Reports From Here:
Orthobiologics Market - https://market.us/report/orthobiologics-market/
Surgical Microscopes Market - https://market.us/report/surgical-microscope-market/
Smart Inhalers Market - https://market.us/report/smart-inhalers-market/
Digital Biomarkers Market - https://market.us/report/digital-biomarkers-market/
Precision Oncology Market - https://market.us/report/precision-oncology-market/
Breast Cancer Market - https://market.us/report/breast-cancer-market/
Medical Carts Market - https://market.us/report/medical-carts-market/
Hair Transplant Market - https://market.us/report/hair-transplant-market/
Lawrence John
Prudour
+91 91308 55334
Lawrence@prudour.com
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release