FDA Altis 522 Study 3-year Data: 8% Risk of Leg Pain vs. 0.6% in Full-length Slings
Study shows that Altis is associated with higher rates of leg pain and an increased risk of pain that requires analgesia
SANTA BARBARA, CA, UNITED STATES, September 25, 2024 /EINPresswire.com/ -- “(Full-length mid-urethral slings) are associated with significant complications, including bladder perforation and vessel injuries with RMUS (retropubic slings), and postoperative groin and thigh pain with TMUS (transobturator slings). To minimize these complications, a new generation of slings emerged: single incision slings (SIS),” states Dr. Le Mai Tu, Neurourol Urodyn. 2023; 42: 1722-1732.
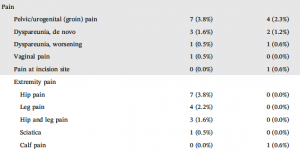
Dr. Greg Vigna, national midurethral sling attorney states, “Unfortunately, the Altis single incision sling data from the Altis 522 study does not show less pain. It showed more pain. The Altis 522 study results revealed that lower extremity pain was reported in 15 out of 184 (8%) patients in the Altis group, and 1 out of 171 patients in the full-length mid-urethral sling group. Despite the Altis sling not entering the leg, it is associated with leg pain.”
What did Dr. Le Mai Tu report in “Management of Female Stress Urinary Incontinence with Single-incision Mini-sling (Altis): 36-month Multicenter Outcomes” published in Neurourol Urodyn. 2023; 42: 1722-1732?
What did the Altis 522 study show?
Dr. Vigna continues, “The Altis 522 study shows that there is an increased frequency of pelvic and leg pain when compared with full-length mid-urethral slings. Coloplast has demonstrated that its version of the new generation does not ‘minimize these complications’ as the author may have expected or hoped.”
Read Dr. Tu’s study: https://onlinelibrary.wiley.com/doi/full/10.1002/nau.25256
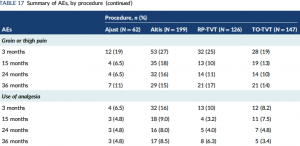
Dr. Vigna adds, “The SIMS (single-incision mini-slings versus standard synthetic mid-urethral slings) study from England was a three-year, randomized control study from England which revealed that women implanted with the Altis had an increased frequency of pain that required the use of analgesia at all times when compared to the women implanted with the Bard Ajust (mini-sling) or full-length mid-urethral slings.”
What did the SIMS data show?
Read the SIMS trial: https://www.ncbi.nlm.nih.gov/books/NBK587586/pdf/Bookshelf_NBK587586.pdf
Dr. Vigna concludes, “The Altis is associated with higher rates of leg pain, which appears to be an unexpected outcome. There also appears to be an increased risk of pain that requires analgesia, which again appears to be an unexpected outcome.”
Red Flag Warning Symptoms of neurological injury or myofascial pain caused by the Coloplast Altis and Aris transobturator slings, and Boston Scientific Solyx and Obtryx transobturator slings include:
1) Groin pain
2) Hip pain
3) Inability to wear tight pants
4) Clitoral pain or numbness
5) Severe pain that makes vaginal penetration impossible
6) Tailbone pain
7) Anorectal pain
8) Painful bladder
9) Pain with sitting
Click here for a free book on Vaginal Mesh Pain.
Dr. Vigna is a California and Washington DC lawyer who focuses on catastrophic injuries and neurological injuries caused by mid-urethral slings, including pudendal neuralgia, obturator neuralgia, ilioinguinal neuralgia, and complex regional pain syndrome. Ben Martin is a national pharmaceutical injury attorney in Dallas, Texas. The lawyers represent women in courts across the country.
Greg Vigna, MD, JD
Vigna Law Group
+1 800-761-9206
email us here
Visit us on social media:
Facebook
X
LinkedIn
Distribution channels: Business & Economy, Education, Healthcare & Pharmaceuticals Industry, Law, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release